Computer System Validation (CSV) is essential for pharmaceutical companies using digital tools in regulated environments. With increasing focus on data integrity, audit trails, and regulatory compliance, having a strong CSV framework is now a must.
Auxochromofours provides end-to-end support through its https://www.auxochromofours.com/services/computer-system-validation-csv/ Computer System Validation (CSV) services, helping organizations validate critical systems used across quality, manufacturing, and regulatory functions.
As pharma companies move toward digital quality systems and electronic records, CSV ensures that software used in GxP environments performs consistently and meets compliance requirements.
Why CSV Is Critical for Pharma Compliance
Regulatory bodies expect pharmaceutical companies to maintain reliable, compliant systems that protect data integrity and patient safety.
Any gaps in validation can result in inspection findings, audit observations, and even regulatory actions. CSV supports compliance with global standards like FDA 21 CFR Part 11 and EU GMP Annex 11 while strengthening broader quality frameworks such as in vitro research services in drug discovery, helping organizations maintain reliable, compliant data across regulated research workflows.
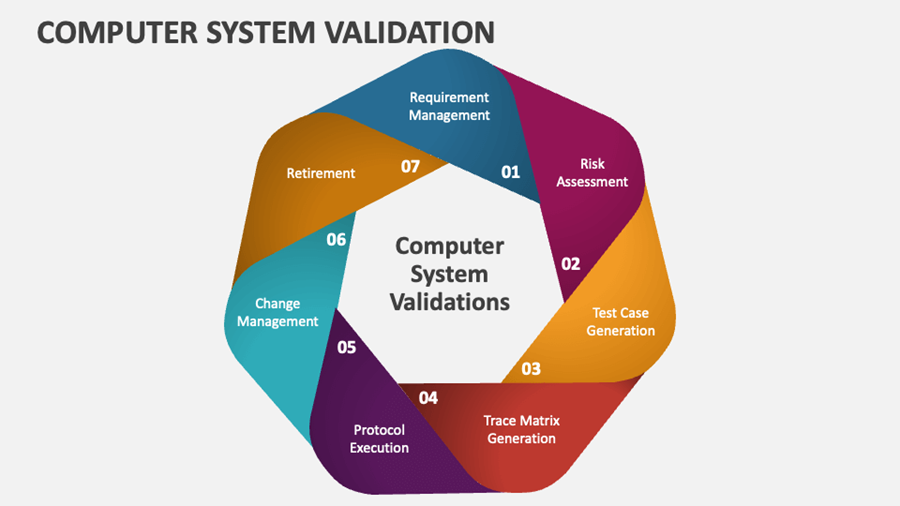
CSV Lifecycle: How It Works in Practice
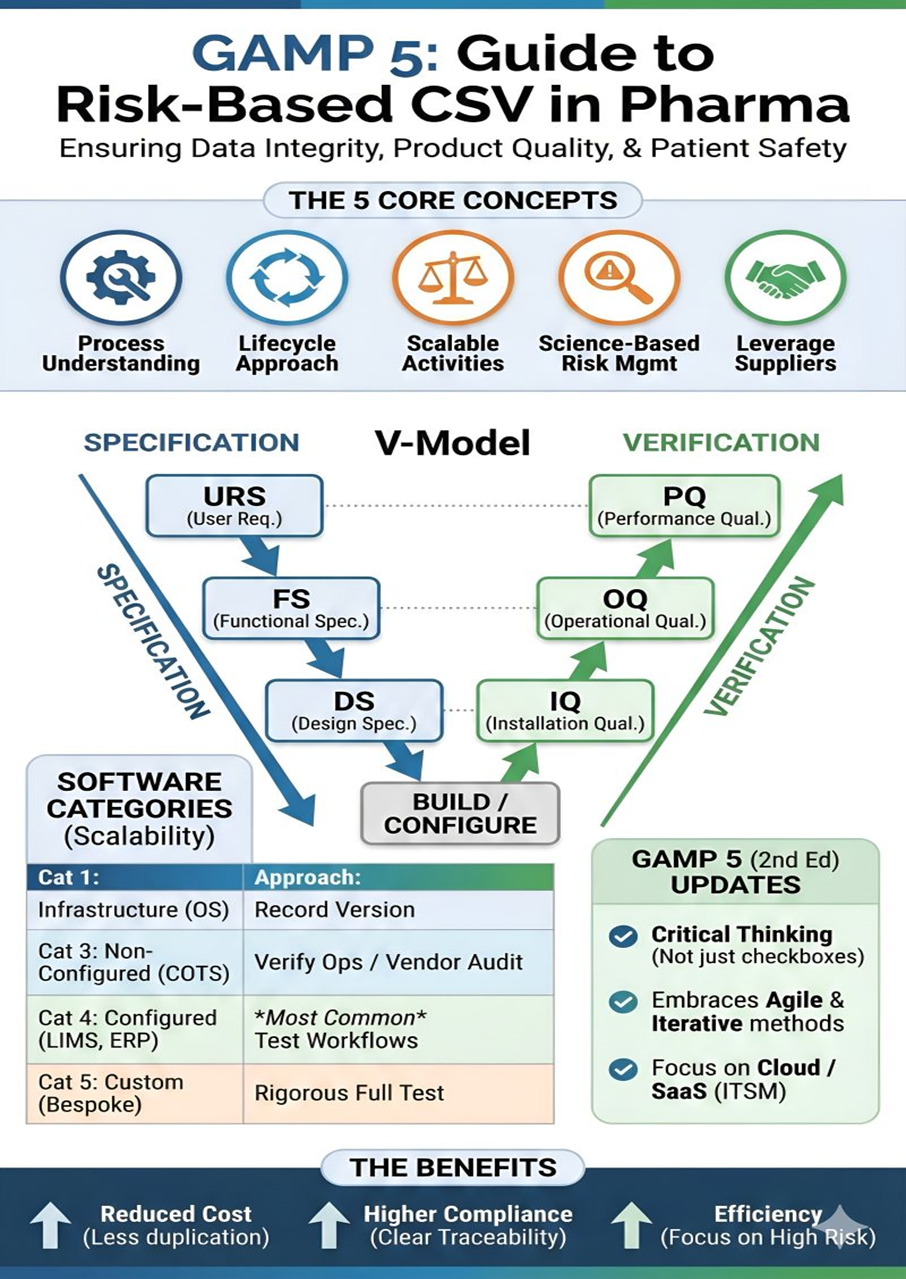
The CSV lifecycle includes planning, requirement definition, system testing, and ongoing change control. A risk-based validation approach ensures that critical systems impacting product quality and patient safety receive deeper validation. Strong documentation practices play a major role in audit readiness.
Many pharma teams align CSV documentation with regulatory data submission standards such as SEND datasets review by regulatory authorities to ensure validated systems support compliant data formats, clear traceability, and smooth regulatory inspections.
Common CSV Challenges in Pharma
Organizations often face issues such as legacy system validation, documentation overload, and poor change control processes. These challenges can weaken overall compliance programs if not addressed properly.
Pharma compliance management systems help teams streamline audits and maintain continuous compliance. CSV becomes far more effective when integrated into broader compliance frameworks such as understanding CRFs in clinical trials, enabling organizations to strengthen documentation, improve audit readiness, and maintain regulatory alignment.
CSV and Regulatory Strategy Alignment
CSV should not be treated as a one-time activity. It works best when aligned with long-term regulatory planning and digital transformation goals.
Many organizations link CSV frameworks with computer system validation in pharma to stay inspection-ready while adopting new digital tools.
FAQs on Computer System Validation (CSV) in Pharma
1. What is Computer System Validation (CSV) in pharma?
Computer System Validation ensures that software and digital systems used in pharma work correctly and meet regulatory requirements.
2. Why is CSV required for regulatory compliance?
CSV is required to ensure data integrity, system reliability, and compliance with regulations like FDA 21 CFR Part 11 and EU GMP Annex 11.
3. Which systems in pharma require CSV?
Systems such as LIMS, MES, eQMS, clinical trial systems, manufacturing software, and regulatory submission platforms require validation.
4. What is a risk-based approach to CSV?
A risk-based approach focuses validation efforts more on systems that impact patient safety, product quality, and data integrity.
5. How often should CSV be performed?
CSV should be done before system go-live and repeated whenever there are system updates, changes, or major regulatory updates.
6. What happens if CSV is not properly implemented?
Poor CSV can lead to audit findings, warning letters, data integrity issues, and delays in regulatory approvals.
7. Is CSV required for cloud-based and SaaS systems?
Yes, cloud and SaaS systems used in GxP environments must also be validated to meet compliance requirements.
8. Who is responsible for CSV in pharma companies?
Quality, IT, and regulatory teams usually share responsibility for CSV, often supported by specialized CSV service providers